Introduction
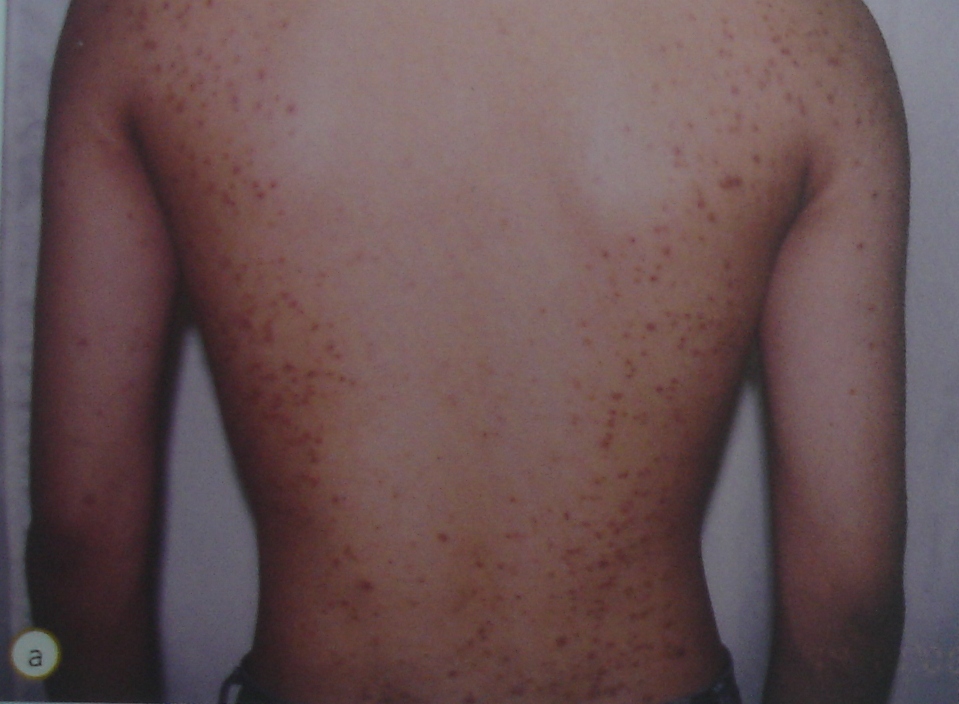
Image 1: Rash caused by a health supplement adulterated with steroid
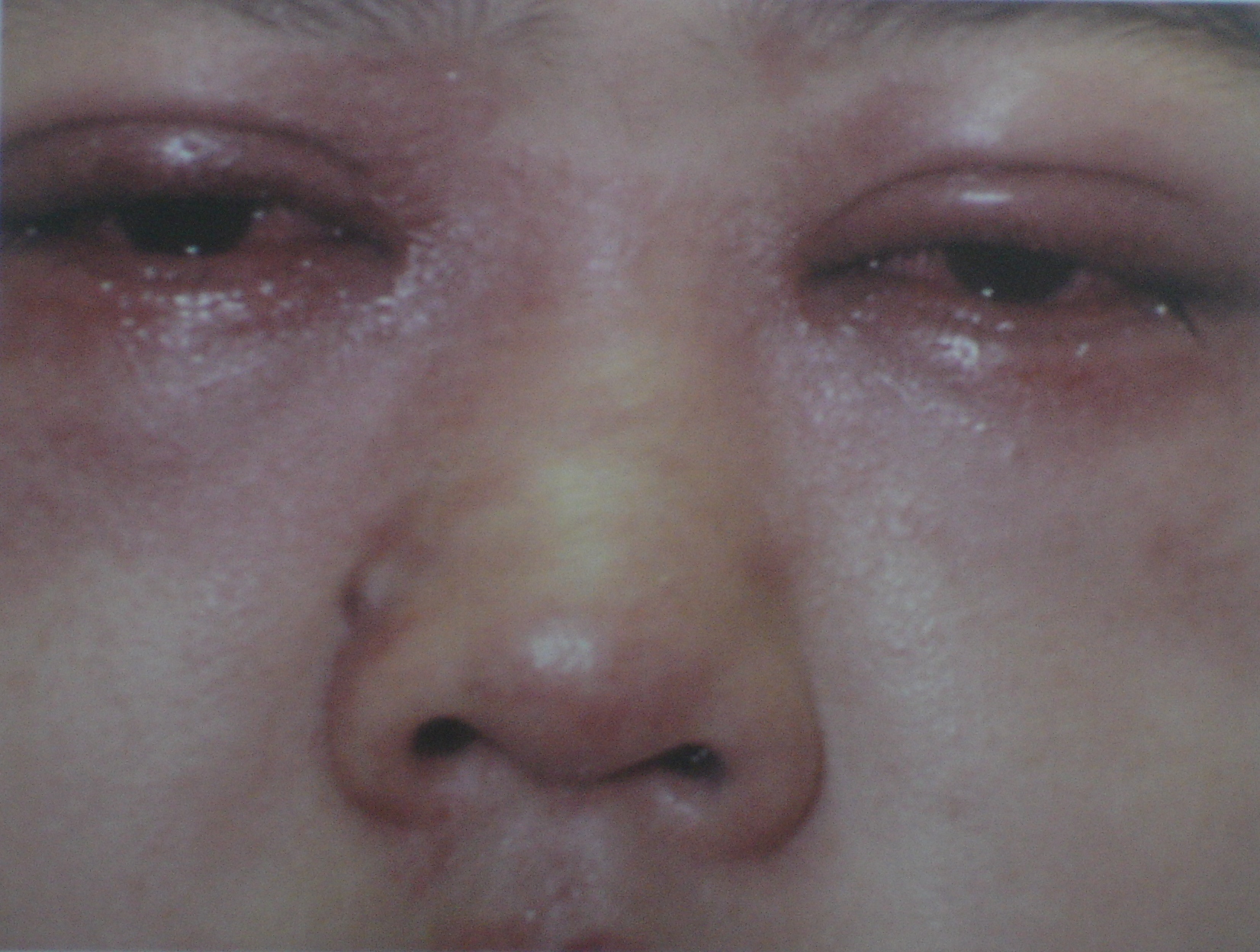
Image 2: Swelling of the eyes after using eye drops1 “That painkiller made my face puff up like a balloon!” “My neighbour’s kidneys were destroyed because he took a blood pressure medicine.”
We have all heard horror stories on the bad effects of medications. It is a fact. All medications come with the risk of adverse or unwanted effects. The point is to ensure the benefit of taking the medicine is more than the risk of adverse effects.
Adverse Drug Reaction or Side Effect?
What are the differences between these terms? Generally, the terms are used interchangeably, with side effects used as the ‘layman’ term.
There are subtle differences between Adverse Drug Reactions (ADRs) and side effects. These are laid out in the table below. For the purpose of this article, the term ‘ADR’ will be used to refer to all adverse effects.
Table 1: Comparison between Side Effects and Adverse Drug Reactions2
|
Side Effect
|
Adverse Drug Reaction
|
|---|---|
| Unintended effect of the medicine Occuring at normal dose used |
|
| Related to the way the medicine works | Not related to the drug action |
| May be beneficial or harmful | Harmful (noxious) |
| Can be expected | May be unexpected and inexplicable |
| e.g. Aspirin (blood thinning medicine) may cause nose bleeds or bruising | e.g. Allopurinol (a treatment for gout) can cause serious skin rashes |
Are Medicines Dangerous?
No. Think of it this way. If you must choose one, which would you pick:
- ankle swelling, or
- a massive stroke?
Most people would naturally choose (a).
These are the kind of decisions your doctor and pharmacist are faced with when deciding whether to give you a medicine. This is known as the risk-benefit analysis.
By taking the blood pressure medicine amlodipine, you may develop ankle swelling. However, your blood pressure will be controlled and you avoid getting a massive stroke. Therefore the benefit outweighs the risk, and your doctor may start you on this medicine.
Registered medicines are continually monitored by regulatory authorities such as the National Pharmaceutical Control Bureau (NPCB) in Malaysia, the United States Food and Drugs Administration (US FDA), and the Therapeutic Goods Administration (TGA) in Australia. Efforts are taken to ensure medicinal products available on the market are safe, with benefits outweighing the risks.
‘Natural’ Does Not Always Mean Safe
Even traditional and complementary medicines such as herbal products may cause ADRs. This may be due to the herbal/natural component itself, or other ingredients in the product.
In 2012 alone, 151 ADR reports involving traditional products were received in Malaysia. More than 70% involved non-registered products3. Many of the products were found to be adulterated with prescription drugs, which could endanger the user.
Therefore, it is very important to check that all products used are registered with the Drug Control Authority, Malaysia www.bpfk.gov.my). Consult your doctor or pharmacist before taking any product or dietary supplement.
Why Didn’t My Doctor Tell Me This Could Happen?
Have a look inside your medication packaging. See that piece of paper? That is called the package insert. It contains detailed information about your medication for healthcare professionals. If you look under the heading “Undesirable Effects”, you will probably see a very long list of medical terms. Every drug may cause various ADRs, ranging from the very common to very rare.
Your doctor will usually inform you about the common ADRs you may have to a drug. However, it is almost impossible to inform a patient of all the possible ADRs of a drug. Some ADRs may be so rare that they are not even written in the package insert.
Some doctors and pharmacist are reluctant to inform patients about possible ADRs for fear they will develop imaginary reactions. This is called the ‘nocebo’ effect. Several studies have shown that patients complained of ADRs such as pain, itching, or stomach discomfort, even when they were only given sugar tablets (placebo) in place of the real medicine.
The best thing to do is to watch out for any adverse reaction, and consult your pharmacist or doctor if you think you are having an ADR which bothers you.
Keep a close watch especially when:
- you start a new medicine (including vitamins or herbals)
- there is a change in the brand/ colour of the medicine
- the dose is increased
- you are taking many medicines at the same time
So Should I Just Bear It or See My Doctor?
The first rule is DO NOT stop your medicine without consulting your pharmacist or doctor. In some cases, stopping the medicine suddenly may cause more harm than the ADR itself.
ADRs can range from mild to serious reactions, such as slight itching or nausea, to liver failure or Toxic Epidermal Necrolysis (where the skin peels off more than 30% of the body).
When to seek medical advice:
- If you find the ADR troublesome or worrying
- If the ADR affects your daily living
- If you want to stop taking the medicine due to the ADR
Your pharmacist or doctor will be able to tell you if the reaction you are having is due to your medicine or some other cause. They will advise you on the best action to be taken. This may involve changing to another medicine, reducing the amount of medicine you take, or treating the ADR while continuing the medicine which caused it.
Some ADRs will resolve on its their own while the medicine is continued. For instance, many people feel bloated or have diarrhoea when they first start taking the diabetes medicine acarbose. However, this effect usually disappears within two weeks when they continue taking the medicine.
Always ask your doctor or pharmacist to give you name of the medicine which caused the ADR. They may give you a Drug Allergy Card to keep, which you should show your healthcare professional before taking any new medicine.
Image 3: An example of a Drug Allergy Card from the Ministry of Health Malaysia
Have an ADR? Report Now!
Please report to the NPCB if you have an ADR that troubles you. This will help to identify new adverse effects of a medicine and may contribute towards improving the safe use of medicines.
What to report?
Report any symptom that worries you, even if you are not sure it is due to the medicine. It is especially important to report adverse effects which are:
- not listed in the product information leaflet
- serious or interfere with your daily activities
Why is reporting important?
Every report helps us know more about that medicine. The information from your report will be entered into the Malaysian and World Health Organisation (WHO) databases of ADRs.
The reports are analysed to identify any safety problems with medicine. The more reports received, the more likely it is that new or rare ADRs will be noticed fast. If many reports are received regarding a particular medicine, a thorough safety review will be carried out.
How to report?
Image 4: Consumer Reporting Form for Medicine Complaints
Obtain a reporting form from the NPCB website or from your pharmacist. Choose one of the following ways to report:
References
- Source of Images 1 and 2: Ridzwan R, NikSulaiman NM, et al. Problem Orientated Atlas of Dermatology for Primary Care Providers. Selayang Hospital, 2004.
- WHO. International monitoring of adverse reactions to drugs: adverse reaction terminology. WHO Collaborating Centre for International Drug Monitoring, Uppsala, Sweden, 1992.
- The Malaysian National Centre of Adverse Drug Reactions database[accessed March 2014].
- Mitsikostas DD1, Mantonakis L2, Chalarakis N2. Nocebo in clinical trials for depression: a meta-analysis.Psychiatry Res. 2014 Jan 30;215(1):82-6.
- Martina Amanzio, Luca Latini Corazzini, Lene Vase, Fabrizio Benedetti. A systematic review of adverse events in placebo groups of anti-migraine clinical trials. Pain. 2009 Dec;146(3):261-9.
| Last Reviewed | : | 10 June 2014 |
| Translator | : | Rema Panickar a/p Sugunan@Vasanthan |
| Accreditor | : | Rokiah bt. Isahak |