Introduction
If someone falls ill or has an impaired health, the important thing to do is to get medicines. Medicines help to cure disease or restore the normal healthy state.
As defined under the Sales of Drug Act 1952, medicine (drug) is including any materials, products or articles intended or attempted to be used on humans or animals either internally or externally, for medical purposes.
In our country, all the drugs that are used either in hospital or sold in stores pharmacies must be registered with the Ministry of Health (MOH). The purpose of registration is to ensure that medicines used in our country are safe, effective and of quality. Drug registration with the MOH, whether modern or traditional, will safeguard consumers from fraudulent and ineffective or harmful products.
What is prescription medicine?
In pharmaceutical industry, drugs are classified into two main categories: drugs containing scheduled poisons and drugs without scheduled poisons:
- Drugs containing scheduled poisons are drug contains poisons that have been listed in the Poisons Act 1952.
In simple language, this drug is known as controlled medicines. All controlled medicines have a registration number that ends with the letter ‘A’. For example MAL20031234A. - Drugs without scheduled poison is the drug which can be bought in community pharmacy or other premises without the need for a prescription from a doctor or consultation with a pharmacist. Examples of these drugs are vitamins, supplements and traditional medicines. All drugs in this category have a registration number that ends with the letter ‘X, N or T’.
Category of product registration.
Source: Cawangan Penguatkuasa Farmasi Negeri Selangor.
Controlled medicines are classified into two groups, namely a prescription medicines and non-prescription medicines. A prescription medicines can only be supplied to patients after obtaining a prescription from a doctor. Examples of such medicines are drugs for heart disease, high blood pressure and asthma. Generally, prescription medicines are controlled under the Poisons Act 1952. Its advertising is controlled by the Medicine Advertisement Board which was established under the Medicines (Advertisement and Sale) Act 1956.
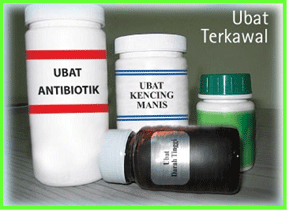
Example of controlled medicines.
Source: Bahagian Penguatkuasa Farmasi KKM
However, the Poisons Act 1952 also gave authorization to the pharmacist to supply medicines without a prescription. A medicine without a prescription is a controlled drug which can be obtained from community pharmacies by meeting a pharmacist on duty without presenting a prescription from a doctor. However, the medicines which can be supplied only limited to certain types of medication such as creams, fever medicine, cough and cold medicine.

Patient who suffer a cough and cold may obtain a medicine without a prescription.
How to get Prescription Medicines?
As mentioned above, prescription medicines can only be supplied to patients after obtaining a prescription from a doctor, thus a prescription medicines can only be obtained from legitimate sources only for example from hospitals, clinics or pharmacies. The doctors are available in these facilities to ensure the medication can be prescribed to patients. While pharmacists will help to monitor the appropriateness of the medicines supplied to patient.
Generally, prescription medicines can only be obtained from:
- Hospital
- Clinic
- Community Pharmacy
- Other registered medical premises with a doctor on duty

A community pharmacy.
Source: http://quezi.com/wp-content/uploads/2009/02/pharmacy.jpg
Why a prescription medicines advertisement is not available in any media?
Medicines advertisements in Malaysia are controlled by the Medicine Advertisement Board who is authorized under the Medicines (Advertisement and Sale) Act 1956 to approve a medicines-related. Medicines advertisements help people to get information about drugs before making decision pertaining to their treatments.
For more information on the regulation of drug advertising, please click here.
Example of prescription medicines advertisement in overseas.
Source: www.fda.gov
In Malaysia, the Medicine Advertisements Board who is responsible for controlling the advertising of prescription medicines has decided that prescription medicines’ advertisements is not approve to be advertised to the public. Therefore, prescription medicines cannot be advertised openly to the public in any media.
The prohibition is aimed at ensuring that prescription drugs are not misused by the public. As prescription medicines can only be provided if a person has obtained a prescription from a doctor, then an openly advertising is not necessary. If a patient requires further information on prescription medicines received, he can get the information directly from the doctor or pharmacist who supplies the medicine.
Similarly, if the doctor or the pharmacist who wish to know any more information on any prescription medication, he can obtain information directly from the drug supplier. In addition, the drug information be able be obtained from the drug label and package insert accompanying each drug supplied.
Medicine label.
Source : http://knowyourmedicine.gov.my/aeimages/Image/Poster_03-Label_Ubat.jpg
Prescription medicines should not been openly advertised in the media because it must be taken with precaution. All prescription medications have specific indications or uses for a particular disease. Therefore, patients who take these medications should be carefully examined by a doctor before giving a prescription drug.
The pharmacist is responsible for providing advice in respect of the correct medication. Besides he needs to identify any interactions or side effects of prescription medicines if patients are taking other medicines along with prescription drugs. Hence, advertising of prescription medicines should not be made public. Advertising in social media and the internet are also not allowed.
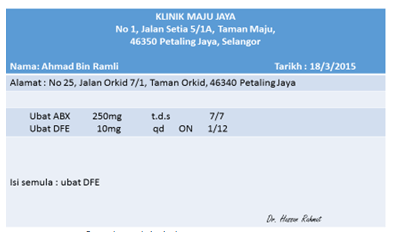
Examples of information that should be included in the prescription, as indicated in the Poisons Act 1952 are below.
An example of prescription.
Exemption
However, there is a flexibility given by the Medicine Advertisement Board for certain conditions, whereby an advertising of prescription medicines to the public is allowed if it is approved by the Medicine Advertisements Board.
Legal provisions also allow prescription medicines advertising if it is intended to provide information to the relevant parties such as doctors, pharmacists, nurses and members of the local authority or the governing body of a hospital and the like as has been provided under the Medicines (Advertisement and Sale) Act 1956. Advertising by the government are also not subject to these rules.
How to get a prescription medicines information?
The users can acquire prescription drug information directly from doctors who prescribed their medicines. The doctor is responsible for ensuring the correct prescription medicines in terms of indications, dosage and cost (cost-effective).
In addition, the user or patient can also ask their pharmacist as regards their medications. Appropriate drug information with respect to the mechanism of action of drugs, use of drugs in the treatment, the recommended dose for the treatment, pharmacokinetics and potential side effects will be obtained by the pharmacist when the medicine is still in its marketing stage.
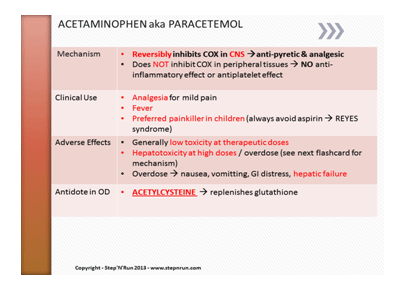
This information will be used to consider the most appropriate medication to a patient. Patients should be provided with adequate information about their medicines in order to ensure they can be personally responsible in taking quality medicines. The figure below shows an example of medicines information.
Example of medicines information.
Source: http://stepnrun.com/images/acetaminophen.PNG
In addition, users also need to take the initiative to read the full information brochure explaining their medication, particularly the goals and methods of use in addition to the potential side effects. Therefore, users or patients are advised to be cautious about buying medicines and please verity the information in the advertisements.
References
- Akta Racun 1952
- Akta Jualan Dadah 1952
- Akta Ubat (Iklan dan Penjualan) 1956
- Sam Wong, ‘Kenali Ubat Anda’, www.konsumerkini.net.my retrieved on 13/2/2015
- ‘Sample Prescription Drug Advertisement’, www.fda.gov retrieved on 17/3/2015
- ‘Cara Pengambilan dan Ciri-ciri Ubat’, ww1.utusan.com.my retrieved on 17/3/2015
- www.pharmacy.gov.my
- http://knowyourmedicine.gov.my
| Last Reviewed | : | 05 June 2015 |
| Writer/Translator | : | Umikalsom bt. Ibrahim |
| Acreditor | : | Muhammad Lukmani bin Ibrahim |