Definition
Blood
Blood is a body fluid with its main function is to transport important substances such as oxygen (O2) and carbon dioxide (CO2). Blood also provides tissues with nutrients, removes waste product, and contains a variety of substances aimed at the immune system defending the body from infection. Endocrine hormones are also distributed through the blood.
Blood Gas
Oxygen is needed for most of the metablolic reactions and carbon dioxide in the blood serves to regulate the balance of acids and bases / alkalis and body respiration .
pH
Scale that measures the acid and alkaline of solution or fluids.
O2 – Oxygen
CO2 – Carbon dioxide
HCO3– – Bicarbonate
Introduction
Blood makes up about 8% of our total body weight, i.e., a person weighing 70 kg (154 lbs) has about 6 liters (10.5 pints) of blood.
The heart pumps blood at a rate of 5.5 L/minute (9.7pints/minute)
Blood is a medium of transport to and from the cells for:
- exchange of food molecules and waste products
- exchange of oxygen and carbon dioxide
- regulation of acid/base balance
- protection against infection
- regulation of body temperature
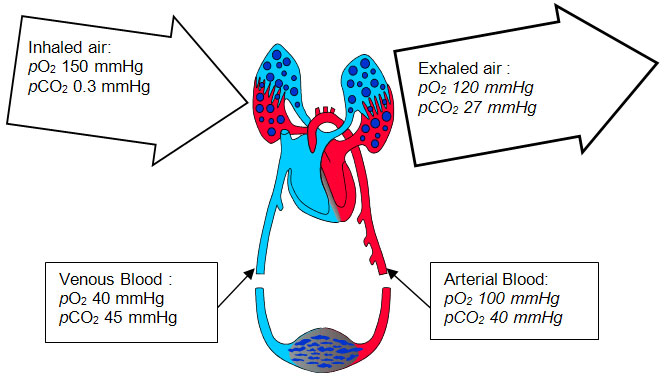
Diagram above shows the respiratory cycle that occurs in the human body and the content of oxygen and carbon dioxide gas which is found in the lungs and blood..
Acid-Base Balance In Blood
pH or acidity of the blood depends on the degree of ions H+ and it is controlled by 3 ways / mechanism; which is the mechanism of buffer / buffer chemistry, respiration and kidney function.
Changes of pH blood occurs through 2 ways :
- Metabolic
Changes in the content of bicarbonate [HCO3–] caused by metabolic disorders. - Respiratory
Changes in CO2 partial pressure (pCO2) caused by respiratory disorders.
Changes in pCO2 and / or HCO3– will lead to changes in blood pH. Acidosis (pH drops below normal) will occur when pCO2 increases and / or bicarbonate decreased, whereas alkalosis occurs when otherwise.
In the blood, the level of acidity is measured in pH units, the value of 1 (acid) to 14 (base / alkaline) with normal or neutral value of 7.. Most processes in the body run normally at physiological pH of 7.4 .
To ensure the pH remains within the normal level, acid base balance in the blood needs to be controlled precisely as little changes in pH can lead to serious effect to the organ or system.
Acid base balance in blood are controlled through 3 ways / mechanism :
- Excessive acid are excreted by renal, mostly in the form of ammonia.
- Buffer in the blood serves as a protector against pH changes that occur suddenly. Bicarbonate is the most important buffer in blood.
- Excretion of CO2 in the lungs
Production of CO2 gas from metabolic processes will be carried by the blood to the lungs for excretion.
Test For Blood Gas
How is it used?
Blood gas measurements are used to evaluate patients oxygenation and acid/base status. They are typically ordered if there are worsening symptoms of
- an acid/base imbalance,
- difficulty of breathing, or
- shortness of breath.
Blood gases may be ordered along with other tests, such as
- Electrolytes test to determine if an electrolyte imbalance is present,
- Glucose test to evaluate blood sugar concentrations,
- Urea and Creatinine tests to evaluate kidney function.
For patients who undergo supplemental oxygen therapy, blood gases may be used to monitor the effectiveness of that treatment.
When the test is performed?
Blood gas tests are performed for patients with symptoms of :
- difficulty breathing,
- shortness of breath,
- nausea
- vomiting
Blood gas measurements may be ordered for patients with :
- Head or neck trauma, injuries that may affect breathing.
- Renal disease and respiration disorders.
What was measured?
The measured parameters are :
- Partial pressure of oxygen (pO2)
- Partial pressure of carbon dioxide (pCO2)
- pH
- Bicarbonate (HCO3–)
- Oxygen saturation (O2)
Procedure For Blood Sampling
- Preparation for patientsThe respiratory condition of the patient should be stable.
-
The patient should preferably be in a steady state of ventilation before and during the collection of blood sample.
-
The patient should be informed about the procedure in order to avoid unnecessary anxiety. If the patient hyperventilation due to anxiety, for example pH and blood gases may be affected.
-
- Tube usedSyringe with anti-coagulant
- Sample typeThere are two types of sample for blood gas analysis :
- Arterial blood gasThe most preferred and recommended type of blood for pH/blood gas analysis
– It gives the best information about the oxygen uptake in the lungs and oxygen transport.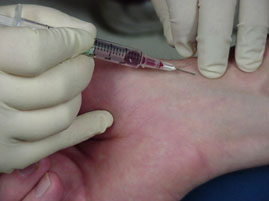
Picture shown the blood sample taken from arterial blood vessel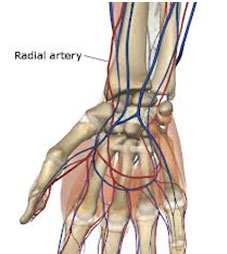
Picture shown the location of arterial blood vessels - Venous Blood GasNot generally recommended for blood gas analysis
– Should not be used to evaluate oxygen status
– Can be used to reflect acid-base status (pH, pCO2, HCO3–).
- Arterial blood gasThe most preferred and recommended type of blood for pH/blood gas analysis
- Procedure of sample collection
- Indicate time of arterial or venous puncture in the request form.
- Use a 1 ml disposable syringe
- If using regular syringe, rinse it by sucking heparin (5000 units per ml) into the syringe and expel excess heparin solution from the syringe.
- If using alcohol to clean the venipuncture site, allow the alcohol to dry completely.
- Draw 1 ml of blood
- Invert the syringe upward and expel all air bubbles.
- Mix well by rolling between palms for 5 seconds
- Specimen Transportation to the laboratory
- Syringe is placed in a specimen plastic bag/ container containing water and ice. Make sure the blood in the syringe is immersed completely in ice water
- The sample must reach the laboratory for analysis in 30 minutes after drawing of blood.
- Analysis of blood gas Analysis of blood gas is performed on analyser with electrode principle.

Example of blood gas analyser
Normal Range
|
Parameter |
Arterial Blood Gas |
Venous Blood Gas |
|
pH |
7.35-7.45 |
7.33-7.43 |
|
pCO2 (mmHg) |
35-45 |
38-51 |
|
pO2 (mmHg) |
71-104 |
30-71 |
|
O2 sat (%) |
95-100 % |
60-85 |
|
HCO3– (mmol/L) |
22-29 |
22-29 |
Table shown normal range for blood gas result.
|
pH result |
Bicarbonate result |
PCO2 result |
Condition |
Common causes |
|
Less than 7.4 |
Low |
Low |
Metabolic acidosis |
Kidney failure, shock, diabetic ketoacidosis |
|
Greater than 7.4 |
High |
High |
Metabolic alkalosis |
Chronic vomiting, low blood potassium |
|
Less than 7.4 |
High |
High |
Respiratory acidosis |
Lung diseases such as pneumonia, |
|
Greater than 7.4 |
Low |
Low |
Respiratory alkalosis |
Hyperventilation, pain, anxiety |
Combinations of results that may be seen in certain conditions are summarized in above table
| Last Reviewed | : | 5 December 2013 |
| Writer | : | Rohaznida bt. Baharudin |







