Endoscopic management of severe uncontrolled asthma in adults
Asthma is a chronic airway disease characterized by inflammation, hyper-responsiveness and narrowing of the airways.
The goal of asthma treatment is to treat the two key components of asthma, i.e. airway inflammation and airway narrowing. The past few years have seen the evolution of asthma treatment leading to better drugs. Although there is still no cure for asthma, several drugs are available to treat and control asthma.
Based on the US data from the Centers for Disease Control and Prevention National Center for Health Statistics in 2005-2009, 24.6 million people were diagnosed with asthma. Of these asthmatics, 12.8 million experienced asthma attacks, there were 1.8 million emergency room visits, 456,000 hospitalizations and 3447 asthma-related deaths. Therefore, the cost of treating asthma is staggering.
The conventional method of treating asthma follows a stepwise approach (Figure 1). This approach depends on asthma severity. In mild asthma, a short-acting bronchodilator would be enough. When symptoms are still uncontrolled, low dose inhaled corticosteroids may be added. If needed, a long-acting bronchodilator or high dose inhaled corticosteroids can be added.
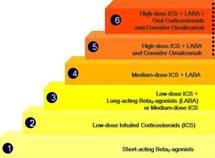
Figure 1
In severe asthma, anti-IgE antibody (Omalizumab) or oral corticosteroids may be required. However, Omalizumab is expensive, is only effective in allergic asthma (asthmatics with high levels of IgE) and has to be administered life-long. Oral corticosteroids have many long-term side-effects. Therefore, alternatives are needed to treat severe uncontrolled asthma.
A new approach to managing asthma was developed a few years ago and it had been approved by the US Food and Drug Administration. This approach targets the airway smooth muscle which undergoes
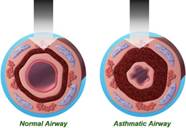 |
hypertrophy in asthma (Figure 2). This novel technology is called Bronchial Thermoplasty (BT). Figure 2
What is BT?
- It is a procedure that delivers thermal energy to the airways via a bronchoscope to reduce excess airway smooth muscle and limits its ability to constrict the airways.
- It is an out-patient hospital procedure performed over 3 treatment sessions, under moderate sedation or general anaesthesia by a trained pulmonologist.
- It is a complementary treatment, not a replacement to current asthma reliever and controller medications
- It is a treatment option that has been shown to increase the level of asthma control and improve quality of life in patients with severe asthma
The principle of BT is the reduction in airway smooth muscle (ASM) which reduces bronchoconstriction, hence reduces asthma exacerbations and improves asthma quality of life (Figure 3).
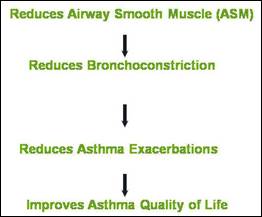
Figure 3
BT is delivered to the patients via the Alair Bronchial Thermoplasty System (Figure 4) which consists of:
- Alair catheter
- Alair Radiofrequency (RF) Controller

Figure 4
The Alair catheter in contact with the airway wall to deliver thermal energy which reduces airway smooth muscle mass and limits its ability to constrict the airways (Figures 5 and 6)
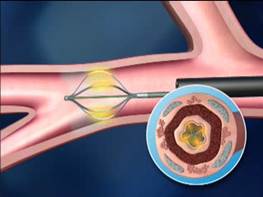
Figure 5
Figure 6
The effectiveness of BT was proven in several clinical studies including 3 randomised controlled trials (AIR, RISA and AIR2 trials).
Results from AIR2 trial demonstrate the benefits of BT which include:
- Improved asthma-related quality of life compared to control
- Improved clinical outcomes compared to control
- 32% decrease in severe exacerbations
- 84% reduction in emergency room visits
- 73% reduction in hospitalization
- 66% less days lost from work, school and other daily activities due to asthma
- Acceptable safety profile
Complications of the procedure:
- No device-related deaths or major adverse events have been reported (e.g. pneumothorax, mechanical ventilation, airway stenosis or focal narrowing)
- More respiratory adverse events are usually observed in the short-term after the procedure typically occurring within one day and resolving within 1-2 weeks with standard care. However, there are fewer respiratory adverse events, hospitalizations and emergency room visits during post-treatment period
Common respiratory adverse events include worsening of asthma, atelectasis, lower respiratory tract infection and haemoptysis. These usually resolve within 1-2 weeks. The patient normally will experience the benefit of BT 2-3 months after the procedure.
Long-term safety profile of BT:
- No deaths have been observed amongst patients treated with this procedure
- Lung function remains stable post-procedure
- No structural changes seen in the lungs (based on CT scan)
Who is appropriate for BT?
- Severe persistent asthma in patients 18 years and older whose asthma is not well controlled with high dose inhaled corticosteroids and long-acting bronchodilators
- Ability to safely undergo bronchoscopy per hospital guidelines
Who is not appropriate for BT?
- Patients who have a pacemaker, internal defibrillator or other implantable electronic device
- Patients who have a known sensitivity to medications required to perform bronchoscopy
- Patients who have previously been treated with the Alair system
BT is performed in 3 separate treatment sessions each scheduled approximately 3 weeks apart. The first session treats the right lower lobe, second session the left lower lobe and final session both upper lobes (Figure 7)
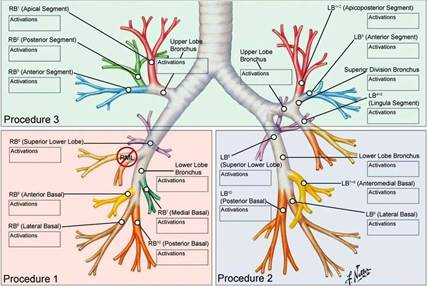
Figure 7
Overview of the procedure:
- Patient is evaluated 1 week prior to BT to verify ability to undergo bronchoscopy
- Prophylactic oral corticosteroids initiated 3 days prior, day of and day after procedure
- Lung function evaluated morning of procedure to assess stability
- Local or general anesthesia administered
- BT catheter introduced through flexible bronchoscope and RF energy applied to airways (approximately 60 activations per treatment session)
- Each treatment session completed within 40-60 minutes
Post-procedure/follow up:
- Patient monitored for 2-4 hours post-procedure
- Patient discharged from hospital same day if:
- Lung function stable within 80% of pre-procedure post bronchodilator FEV1
- Patient stable, able to take liquids, feeling well, adequate mental status
- Prophylactic oral corticosteroids continued 1 day after procedure
- Patient contacted via phone at 1, 2 and 7 days to assess post-procedure status
- Clinic visit at 2 to 3 weeks to assess clinical stability and schedule subsequent BT procedures as appropriate
- Individual patient results are communicated to referring physician as appropriate
- Patient returns to care of primary asthma physician for long term asthma management following BT
BT is now available in Malaysia. Please speak to your doctor for information.
To view the procedure, please click this YouTube link: http://www.youtube.com/watch?v=vCWlXLcuQmY&sns=em
Further information is also available at: http://www.btforasthma.com/
| Last Reviewed | : | 10 November 2014 |
| Writer | : | Dr. Jamalul Azizi b. Abdul Rahaman |
| Accreditor | : | Dr. Norhaya bt. Mohd Razali |







