Introduction
Medicines advertisement control in Malaysia is different from the United Kingdom. In Malaysia, the system applies are approval from the Medicine Advertisements Board (MAB) whereas in the United Kingdom they are practicing self-regulation control.
The following are the comparison of medicine advertisements control between Malaysia and the United Kingdom:
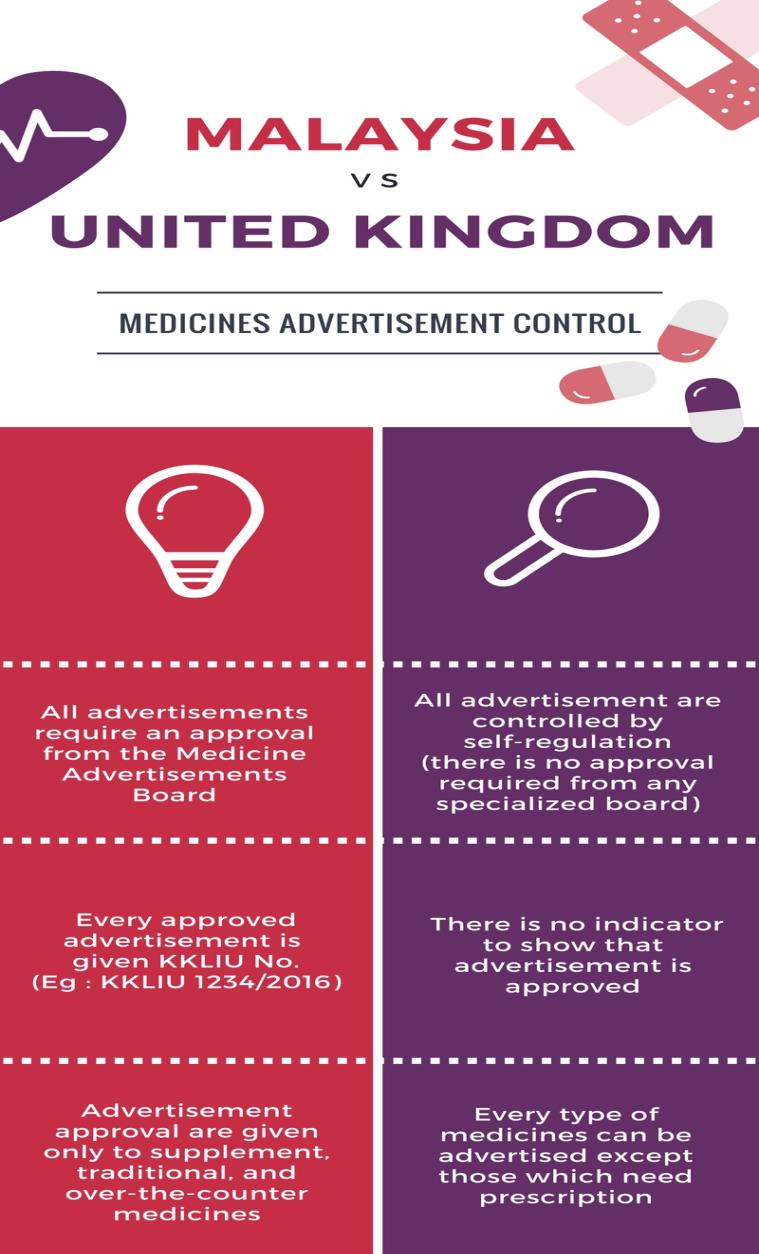
In self-regulation approach, the responsibility to ensure medicine advertisement is abiding the law is not only falls to the hand of the authorities but also shared among agencies and associations concerned. This means that if a member of association is violating a code that has been assigned, action will be taken by the agencies towards the offending member.
The Role of Medicine Advertisements Board
The role and tasks of the MAB are as follows:
- Approve and reject any medicine advertisements application in any media
- Withdrawal or cancellation of approved advertisement whenever necessary
- Approve/amend any guidelines pertaining to advertisement

On the other hand, in UK, all advertisements do not require any approval from the authorities. However, all advertisement must follow the guidelines and act that are enforced by the UK authorities. Self-regulation control is the core to regulate of medicine advertisement in UK. This is due to the punishment is higher compared to the punishment under the Medicines (Advertisement and Sale) Act 1956. Apart from the punishment sentenced, the authority has the power to issue an order for rectified statement. This means the advertiser must release a corrective statement for the misleading advertisement that has been spread.
In addition, even though the advertisement does not require an approval but there are a few conditions which require an evaluation from the authorities:
- Approval of new drugs
- Product which need a re-classification such as drugs which require a prescription by a pharmacist
- Product advertisement which has been taken action for infringing the code/law
Besides that, the UK authorities also have the power to issue an order as follows especially to those involving a risk to the general health:
- Issue an order to make amendment of the advertisement
- Withdrawal of the advertisement
- Issue an order to the advertiser to release a rectified statement
- Submission of new advertisement for approval
Advice to the consumer
Consumers are advised to make sure all medicine advertisements carried an approval number from the MAB. Approved advertisement has been evaluated by MAB. The aim is to make sure all the information are true and correct according to the evidence given during the advertisement application process.

Make sure registered medicine/pharmaceutical product have a MAL registration number along with a hologram sticker and its advertisement is approved by the MAB. Realizing the fact that consumers need information, the Pharmaceutical Services Division has created a dedicated webpage to give information concerning the risk of purchasing medicine online. Please go to http://www.pharmacy.gov.my/v2/ms/website/waspada-belian-ubat-online for tips/guidelines/e-posters and videos.
References
- Medicines (Advertisement & Sale) Act 1956
- The Blue Guide, Advertising and Promotiono Medicines in the UK, Third Edition (2014)
- www.pharmacy.gov.my
| Last Reviewed | : | 13 Oktober 2016 |
| Writer/Translator | : | Normawati bt. Mohamed Noor |
| Accreditor | : | Umikalsom bt. Ibrahim |







