Registration of Pharmaceutical Products in Malaysia
Ministry of Health Malaysia always strive to ensure all pharmaceutical and healthcare products in the market are off good quality, safe, efficacious and regulated according to related laws. Therefore, all pharmaceutical products including health supplements and traditional products should be registered with Drug Control Authority (DCA), Ministry of Health Malaysia (MOH) before it can be marketed in Malaysia.
Products that are registered with DCA are of good quality and safe to be taken by the public. Each registered pharmaceutical product are assigned with a specific registration number that need to be printed on the label or packaging of the product. The registration number starts with ‘MAL’, followed by 8 numbers and it ends with alphabets, for example MAL 20001564T.
However, market monitoring by Pharmaceutical Services Division, MOH found that there are pharmaceutical products with fake registration number or using registration number of other products. This is because registration number is easily copied and users found it difficult to confirm its authenticity during purchase.
To overcome this problem and to improve the security and identity of products registered with DCA, the Ministry has taken a proactive approach. This is by introducing and requiring the use of MeditagTM Hologram security sticker on all pharmaceutical products. This hologram has many security features which can be observed by the user and pharmacy enforcement officer. The MeditagTM Hologram security sticker have been put into use since 1st of May 2005.

Picture 1: Product registered with DCA has 2 features which are registration number and MeditagTM Hologram security sticker.
Introduction to MeditagTM Hologram Security Sticker
MeditagTM hologram is managed by only one (1) appointed company to supply the hologram to manufacturer with GMP (Good Manufacturing Practice) license or to importer which has been licensed by MOH. This is to ensure the MeditagTM hologram is not misused by irresponsible parties.
The hologram label sticker has high security feature and each one is given a serial number. On the 1st of September 2006, the first version of hologram has been replaced by a second version which have enhance security features as additional measures. It may help consumers to identify registered pharmaceutical products. In addition the production of counterfeit holographic sticker may be prevented. The use of MeditagTM hologram successfully helps the pharmacy enforcement officers to take action against pharmaceutical products that are not registered with MOH.
Current MeditagTM Hologram Security Sticker (2012)
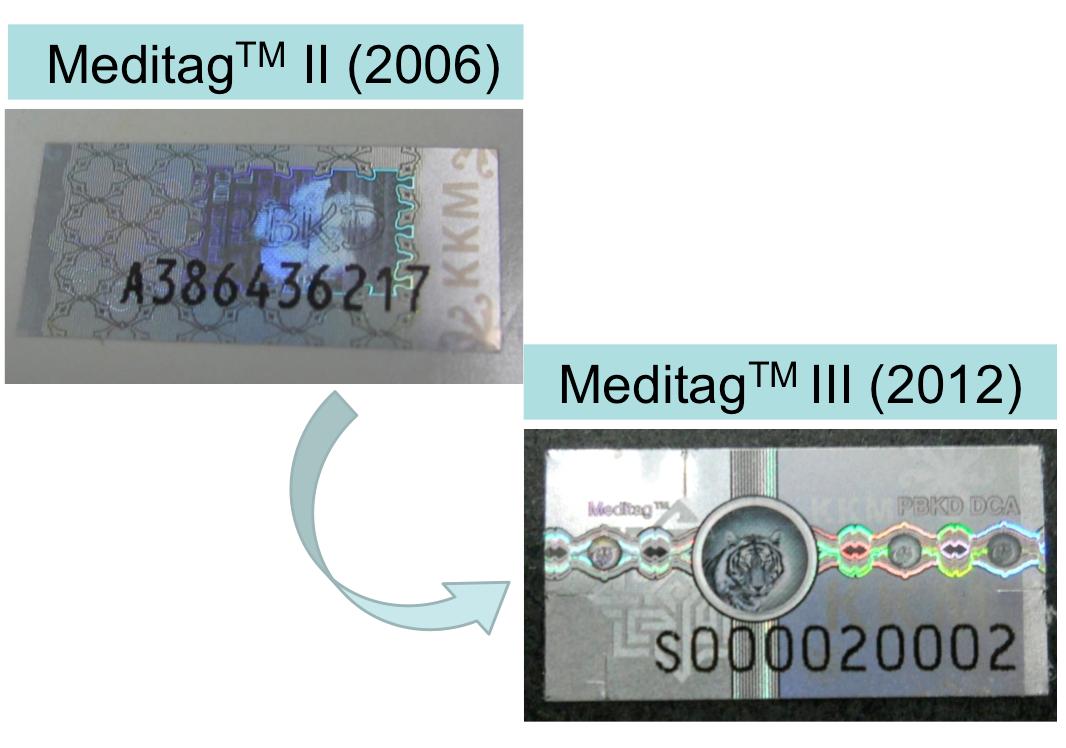
In line with the development of science and technology and also in addition to further enhance the security features in preventing counterfeiting; the third hologram version (Meditag III) has been introduced starting 1st November 2012. The new hologram is equipped with current security features and it is more users friendly.
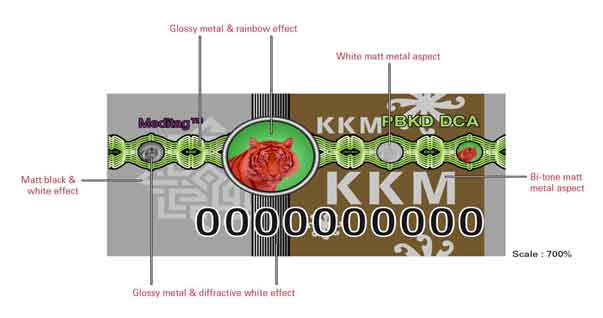
Picture 2: Enhancement of security features on hologram MeditagTM III in line with the current development of science and technology
To ease the user in verifying the hologram authenticity MOH has provided a tool called Meditag decoder to all licensed pharmacy premises and Pharmacy Enforcement Division at each state. It is compulsory to place the tool on the counter of pharmacy premises so that it can be easily used to verify the authenticity of the product. With this, a user can also get advice on medicines from the pharmacist.
Picture 3: Check the authenticity of your product by using Meditag™Decoder available at the nearest pharmacy.
How to Use Hologram MeditagTM Decoder to verify the authenticity of MeditagTM hologram label
MeditagTM II (2006)
|
|
||
| The view before decoder slide is moved – the letter KKM is invisible | Move decoder slide upward – the letter KKM is visible |
MeditagTM III (2012)

MeditagTM Checker Application
In line with the current technology development, MOH took the initiative to introduce the smartphone MeditagTM Checker application. The application can be used to identify the features of authentic MeditagTM hologram on pharmaceutical products. Users can download the MeditagTMChecker application for free through Google Playstore for android system and App Store for iOS system.
Picture 4: MeditagTM Checker application can be downloaded for free by Android or iOS smartphone user.
Users Role
Users are advised to use pharmaceutical products that have been registered with MOH and to always check the authenticity of the products purchased. Avoid from using products that are not registered with MOH or suspicious products. If there is any suspicion, questions or complaints on unregistered products, users can contact MOH Pharmaceutical Services Division at 03-78413200 or visit moh.stab.gov.my
References
- Malaysian Laws On Poisons And Sale Of Drugs. International Law Book Services. Kuala Lumpur. 2006.
- http://www.pharmacy.gov.my
- http://www.knowyourmedicine.gov.my
| Last Reviewed | : | 24 October 2016 |
| Translator | : | Nor Izyani bt. Hanafi |
| Accreditor | : | Munira bt. Muhammad |