Introduction
Oocyte identification is the process of identification oocyte during oocyte retrieval. Oocyte retrieval is a technique used in in vitro fertilization in order to look for, and pick up oocyte-cumulus complexes (COC) from the follicles of the ovaries, enabling fertilization outside the body. Patients receive hormonal stimulation in order to retrieve multiple mature oocytes. Currently, oocyte retrieval based on the transvaginal aspiration guided by a vaginal ultrasound is universally applied for IVF treatment. Two of the most crucial factors that need to be strictly controlled during the procedure of oocyte retrieval are temperature and pH.
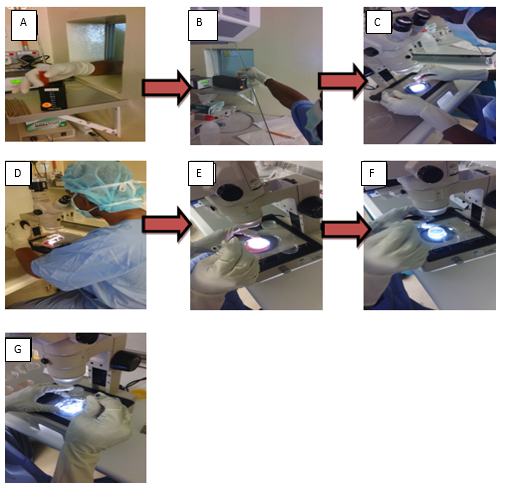
Procedure Of Oocyte Identification(Oi)
- It is very important to prepare Oocyte culture dish after oocyte identification, Oocyte culture dish after ICSI, collection media, and oil a day before OI and pre-warm any disposables that come in direct or indirect contact with the retrieved oocytes to 37 0
- Examined the suction pump and set the suction pressure to -121mmHg and the block heater at appropriate temperature (37± 1oC).
- Verified the patient and husband name and also their identification number.
- Switch off the air conditioner to make sure the room temperature is appropriate preferably 26-270
- Procedure starts once the clinicians inserts the aspiration needle and aspirate follicle fluid contain oocyte from the Ovary using suction pump with the help of ultrasound probe. Collected fluid will be directly transferred from aspiration needle into the collection tube.
- The collection tube is hand over to the embryologist for oocyte identification under microscope. Once oocyte is identified, wash the collected oocyte with collection media, grade them and inform clinician number of oocyte collected and their grade.
- After the procedure is finished, transfer them into culture dish with media and culture them for 4 hours in the incubator equipped with Tri-gas (5% oxygen, 6% carbon dioxide and 89% nitrogen) for further maturation.
Notes: Rinse the oocyte with oocyte culture media since the media used to collect and culture the oocyte is different.
Ocyte Grading During Oi
Oocytes are surrounded by the cumulus and corona cells (COC) at the time of collection (Figure 2A). Under the stereomicroscope, a typical mature preovulatory COC displays an expanded radiating corona surrounded by the loose mass of cumulus cells, macroscopically visible (Figure 2B). During oocyte identification pre-evaluation of oocyte morphology depends on oocyte size and colour. Poor quality oocyte usually smaller in size or dark in colour. Oocyte quality is a limiting factor in female fertility. It is believed that the developmental fate of the embryo is largely determined by the quality of the oocyte. Aspects that may influence oocyte quality can be subdivided into morphological, cellular, and molecular. In routine IVF practice, the quality of oocytes is mostly determined by its appearance and its performance after fertilization. The cellular and molecular aspects that may determine oocyte quality are not yet used in daily routine. Morphological anomalies are frequently observed in human oocytes. After IVF, 13 % of the unfertilized oocytes show morphological abnormalities (Van Blerkom and Henry, 1992).
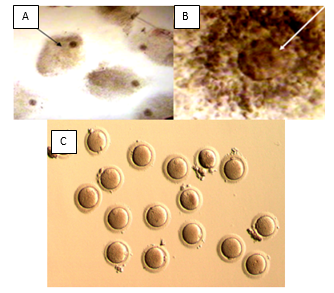
Oocyte Culture
Cumulus contain oocytes usually further culture for 4 hours after oocyte Identification for further maturation of collected oocytes. Oocytes are extremely sensitive to changes in temperature. Temperature fluctuations have detrimental effects for the meiotic spindle. Interference such as disorganization of microtubules within the spindle, a decreased density of microtubules or a lack of microtubules, can disturb the correct segregation of the chromosomes (Pickering et al., 1990; Wang et al., 2001). The maintenance of 37°C during in vitro manipulation is required. Therefore, all disposables and media that come in direct or indirect contact with the retrieved oocytes are pre-warmed to 37°C. The intracellular pH (pHi) of oocytes is around 7.4 during maturation and fertilization. pH oscillations during oocyte manipulations may have detrimental consequences, because the cytoskeletal dynamics and cellular metabolism are highly dependent on pH (Dale, 1998; Swain and Pool, 2009). In order to avoid pH fluctuations of the external pH and also indirectly the intracellular pH, oocytes are collected in HEPES (4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid) buffered at pH 7.3. Media used for oocyte culture need carbon dioxide to maintain pH. Therefore, media used have to be equilibrated beforehand in an incubator (Figure 3). Changes in osmolarity which result from the evaporation of medium might happen during culture. Changes in osmolarity cause shrinking or swelling of the oocytes. Evaporation can be avoided by covering the media with oil to maintain the culture media osmolarity.

Reference:
- Dale B, Menezo Y, Cohen J, DiMatteo L.,Wilding M. Intracellular pH regulation in the human oocyte.Human reproduction. 1998;13:964-970.
- Pickering SJ, Braude PR , Johnson MH, Cant A, Currie J. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertility steril. 1990;54:102-108.
- Swain JE and Pool TB. New pH-buffering system for media utilised during gamete and embryo manipulations for assisted reproduction.Reproduction Biomedical Online. 2009;18:799-810.
- Van Blerkom J, Henry GH. Oocyte dismorphism and aneuploidy in meiotically mature human oocytes after ovarian stimulation.Human Reproduction. 1992; 7: 379–390.
- Wang WH,Meng L,Hackett RJ,Odenbourg R,Keefe DL. Limited recovery of meiotic spindles in living human oocytes after cooling-rewarming observed using polarized light microscopy. Human reproduction.2001; 16:2374-2378.
| Semakan Akhir: | 20 December 2015 |
| Penulis: | Helen Tang |







