General Principle
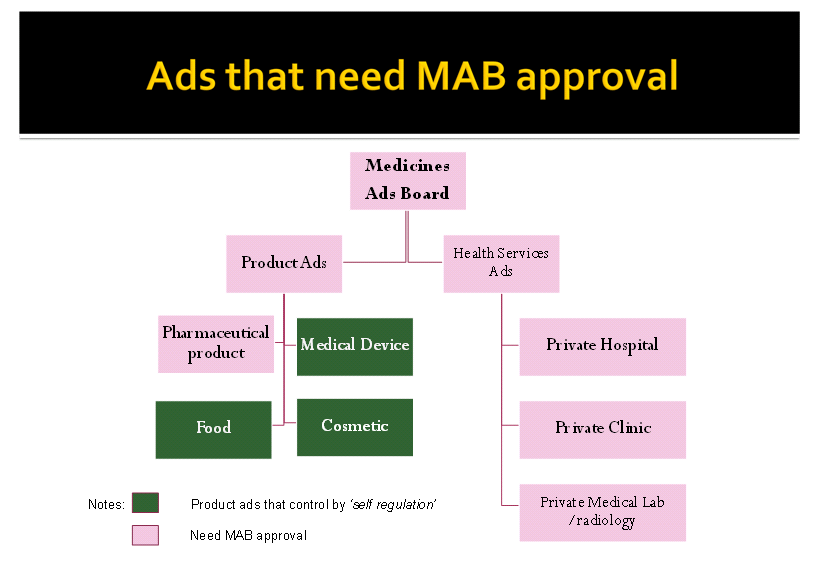
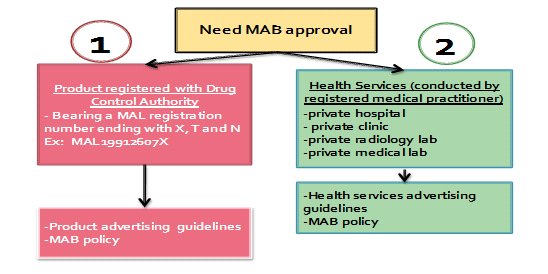
Medicines Advertisement Board (MAB) under Ministry of Health is established under the Medicines Advertisement Board Regulations 1976 is responsible to give approval to an advertisement relating with medicines as well as health facilities. Medicines which are registered by Drug Control Authority need to apply to the MAB for the advertisement to be approved; prior its publication to general public. Those medicines advertisement without MAB approval commit an offence. Health facilities namely hospital/ private clinic and private medical laboratory/radiology advertising their services either for treatment, prevention or/and diagnosis commit an offence if advertise without MAB approval under Section 4A Medicines (Advertisement and Sale) Act 1956.
Though food ads, medical device ads as well as cosmetic ads are self regulated, however they can still be sanctioned under Medicines (Advertisement and Sale) Act 1956 if they advertise the 20 prohibited diseases as stipulated by the Act.
Regulation
Advertisement that requires MAB approval is as below:
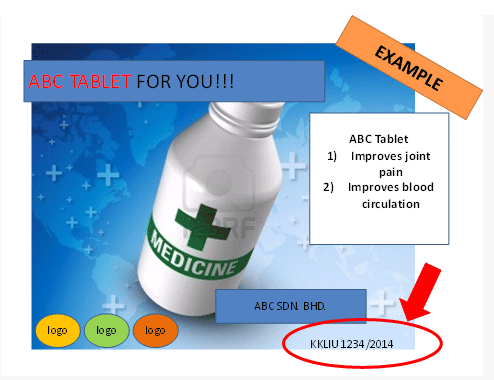
Example
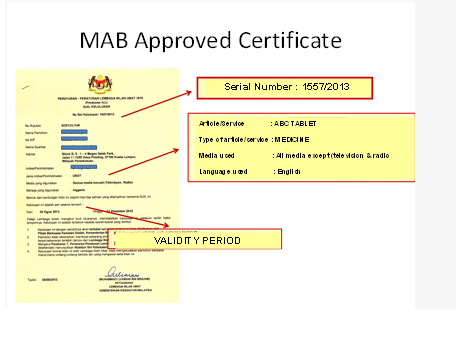
Each approved advertisement need to display the approval number (KKLIU) as shown below. An approved ad will be issued with a certificate together with an approved format. Advertiser is compelled to publish the same format as approved by MAB. The validity period for the approved ads is 3 calendar years.
The role of MAB
- To approve or reject the application
- To withdraw or cancel the approved ads when necessary
- To approve or amend advertising guidelines
Offences
No person shall take part in the publication with regards to prohibited diseases, medicines ads and health services without the approval from MAB. It shall be guilty of an offence under Section 5 Medicine (Advertisement and Sale) Act 1956. For the first offence is to be fined up to maximum RM3000 or up to 1 year imprisonment or both and subsequent offence to be fined up to RM5000 or 2 years imprisonment or both.
Further self-checking
Public can verify the registration status of registered product or notification status of cosmetic through National Pharmaceutical Control Bureau (NPCB) website www.bpfk.gov.my. This portal provides various information relating to the product such as their status, product detail, address of manufacturer, outer packaging and label. Public also can check the withdrawal list of product withdrew from market. Do ensure your medicines are registered; MAL registration number and also affixed with a serialized MeditagTM hologram and the ads is approved by MAB. For more information or query please call Pharmaceutical Services Division at 03-78413200 or email to pharmacy1@moh.gov.my.
References
- Medicines (Advertisement and Sale) Act 1956
- www.pharmacy.gov.my website
- www.bpfk.gov.my website
| Last Reviewed | : | 09 September 2014 |
| Writer/Translator | : | Normawati bt. Mohammed Noor |
| Accreditor | : | Muhammad Lukmani bin Ibrahim |