Definition of Radiation
Radiation is the process of emission and transmission of energy through medium or space in the form of waves or particle nuclei. There are two types of waves, that is mechanical and electromagnetic and two types of particle which are charged and uncharged particle.
Electromagnetic Radiation
Waves can be characterized by the amplitude, wavelength and frequency. The characteristics of the electromagnetic wave are;
- It has an electric and magnetic field component which oscillate in a perpendicular phase to each other and to the direction of energy propagation as shown in Table 1.
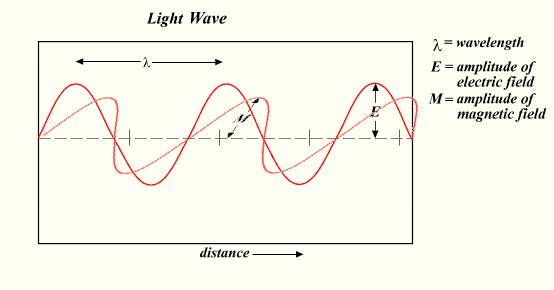
Table 1 : Form of waves - No mass;
- Unaffected by either electrical or magnetic field;
- Has a constant speed in a given medium, 2.998 x 108 m/sec;
- Travel in a straight line and does not require a matter for its propagation.
Electromagnetic radiation can be divided into two (2) types of so called ionising radiation and non-ionizing radiation as shown in the spectrum in table 2. The frequncy for ionising radiation is in the range of 1016 to 1024 hertz, and for non-ionising radiation it is in the range of 1015 to 100 hertz.
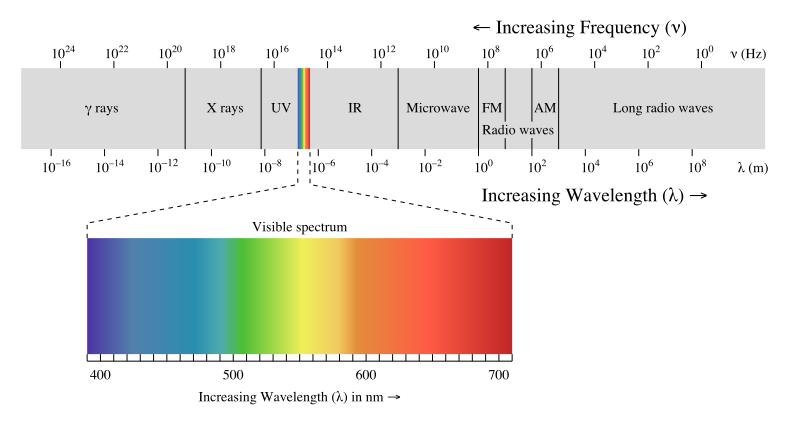
Table 2 : Electromagnetic Radiation Spectrum
Ionising Radiation
Ionizing radiation is an electromagnetic wave with a potential kinetic and quantum energy in certain magnitude to ionise the atom or molecule through atomic interactions. The ionisation will happen when photon is absorbed by the electron in the range of 4-25 eV to remove the electron from the atomic orbital as shown in table 3.
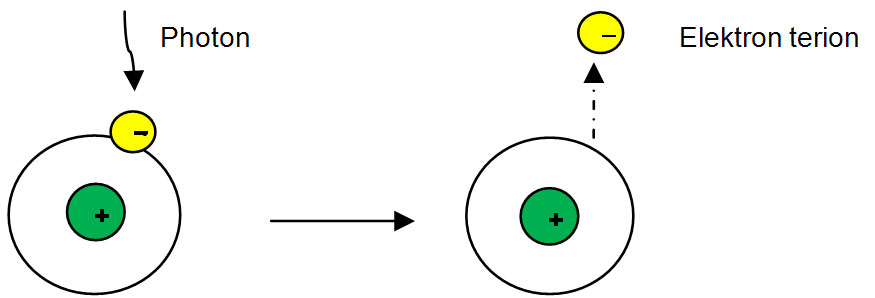
Table 3: Process of ionisation.
Ionising radiation can be categorised as two (2) types :
- Direct ionising radiation deposits energy in the medium through direct Coulomb interactions between the charged particle & orbital electrons of atoms.
- Indirect ionising radiation deposits energy in the medium through a two-step process:
- A charged particle is released in the medium (photons release electrons or positrons, neutrons release protons or heavier ions).
- The released charged particles deposit energy to the medium through Coulomb interactions with orbital electrons of the atoms.
Types of Direct Ionising Radiation
- Alpha ray (?) – ? rays is a positive charged particle composed with two neutrons and two protons. It has a large mass and low penetration which can be stopped by a piece of paper. The ionizing force are higher and can produce about 10 000 to 70 000 ion pair/cm. ? rays exist in the environment which can be produce naturally or human made. ? rays can be emitted by naturally occurring radionuclide such as radon-222, polonium-210, uranium-238, torium-230, plutonium-240 and americium-241.
- Beta ray (?) – ? ray is a negative charged particle which consist of electron. Since ? ray is more lighter and can move faster than ? rays, they are able to penetrate further, and can be stopped by a metal such as aluminium. The ionizing force is lower and can produce only about 60 – 7000 ion pair/cm. ? rays also exist in the environment which can be produced naturally or human made. ? ray can be emitted by naturally occurring radionuclide such as tritium, karbon-14, berilium-10, strontium-90 and bromin-85.
Types of Indirect Ionising Radiation
-
Gamma rays (?) – ? rays can be produced when a photon is emitted through a process inside the nucleus. It can penetrate higher than others and can only can be stopped by several inches of plumbum. Due to its faster speed , the ionising force is lower. ? rays can be emitted from naturally occurring radionuclide such as calcium-40, while cobalt-60 and caesium-137 are produced from man made source.
-
X rays – X rays is emitted from energetic photon by the interaction of charged particle and targeted material. X rays is similar with ? rays but it has different characteristic and source. X rays have lower ionizing force compared to ? and can be stopped by a few millimeters oflead.
-
Neutron – Neutron is a heavy and uncharged particle. It can be emitted as a result of nuclear reactions or fission process, since they cannot by themselves be accelerated electrostatically. Neutron raise the most concern as an external hazard since they have no charge and therefore can travel great distance in air and other media. The neutron ionizing force is lower compared with ? rays and can be stopped by using boron as a shielding material.
The Use of Ionising Radiation
Ionising radiation is more useful in medical purpose as below;
- To diagnose patient by using x-ray machine.
- To control the growth of cancer cells by breaking the atomic binding in the cell which can be used in radioteraphy treatment by using gamma rays from cobalt-60.
- To sterelise the medical equipment such as thermometer, needles, surgical equipments and others.
- To detect the location of blood clots from the blockage of the blood vessels by using natrium-24 radioisotope.
- To localise brain tumour using phosphorus-32 radioisotope.
- To determine the uptake of thyroid activities by using iodin-131 radioisotope.
Ionising radiation can also being used for industrial purpose as below;
- To determine the thicknesses of a material such as in paper manufacturing, metal sheets and plastic sheets, as in cans and packaging.
- To detect the leakage of underground piping by using natrium-24 radioisotope.
- To detect the internal leakage in metal plate by using gamma radiation.
The Effect of Ionising Radiation
Ionising radiation can affect biological cells in the human body if exposed to radiation area certain period of time. The ionising process which take place in the cell can cause damage to the tissue and body organ. The ionising process can also produce chemical reaction and further damage the chemical bond. Individual who are exposed directly to ionising radiation can damage the cels in the body, increase the risk of cancer and cause mutation of future genes. Higher dosage can also cause major damage in the tissue and can cause death after a few weeks of ionizing radiation exposure.
References
- The essential physics of medical imaging by Bushberg JT et. al. Williams & Wilkins, 1994.
- Introduction to Radiological Physics And Radiation Dosimetry by Frank Herbert Attix. University of Wisconsin Medical School, Madison, Wisconsin,1986
- http://ms.wikipedia.org/wiki/Sinaran_pengion
- http://www.who.int/ionizing_radiation/about/what_is_ir/en/
| Last Reviewed | : | 8 October 2014 |
| Writer | : | Haizana bt. Hairuman |
| Accreditor | : | Mohd Khairudin b. Mohamed Samsi |







