Storage And Distribution Of Medicine From Manufacturer/ Importer To User (Cold Chain Medicine)
Medicines like vaccines, blood products and biological mainly serves as lifesaving or prevention of critical illnesses. Mostly, they have specific storage requirements under the cold place ranging from 2-8 degree Celsius. Such cold-chain storage is necessary to prevent damage towards the vaccine caused by heat exposure. However, keeping vaccines too cold can be just as harmful as keeping them too warm, since many vaccines may be damaged by freezing.
Importance of storage
Proper storage and distribution plays an important role in maintaining the quality and effectiveness of medicine when it reaches the end user. In the narrow range of 2-8 degree Celsius, temperature below 2 degree Celsius or above 8 degree Celsius is term as an excursion. Quality and effectiveness of the medicine will be affected if the medicine is not kept in appropriate condition. Critical medicinal products which could be lifesaving may fail to work to its labelled expectation simply due to poor handling and storage practice.
What is cold chain?
Cold chain products are very common on our daily activities especially in the food industry. In the pharmaceutical industry, integrity of the cold chain medicine is equally important. Even if the product is stored out of its recommended condition, the quality of the products will be affected.
There are two essential elements of the cold-chain system to ensure patients that the cold chain medicines are of potent medication. Namely during:
- Transporting and distribution;
- Storage and monitoring
Let’s take vaccine for example; most vaccines have the storage requirement range of 2 – 8 degree Celsius which is usually stored in a cold room or refrigerator. Vaccines are delicate biological substances that will become less effective or even destroyed if they are frozen, allowed to get too hot, or even exposed to direct sunlight or fluorescent light. Vaccines that are destroyed would be unusable as well barely visible; the undesirable effect would be when it is injected, infants and young children are not getting the target immunity after their vaccination.
What are vaccines?
There are many infectious diseases that can results in death or disability of infants and young children. Some of the most dangerous of these are:
- Poliomyelitis
- Measles
- Diphtheria
- Whooping cough
- Tetanus
- Tuberculosis
- Hepatitis B
- Mumps
These diseases have one thing in common – where it can all be prevented by immunization. Immunization is achieved by the administration of a vaccine, produced from an attenuated, inactivated or killed virus or bacteria. A vaccine is normally injected, or in some cases may be given orally. The vaccine will provoke the development of antibodies in the infant, who thus acquires immunity without suffering the disease. In Malaysia, vaccines are introduced through The National Immunisation Programme which provides routine childhood immunisations for various infectious diseases.
How vaccine reaches you?
Compare to food where consumers buy their own food from the retail store, medicinal products like vaccines are commonly handled by specific personnel and finally administered to the patient through health care professionals. Most of the vaccines available in Malaysia are mainly imported from overseas; the handling of the vaccine goes through many stages as illustrated in the image below:
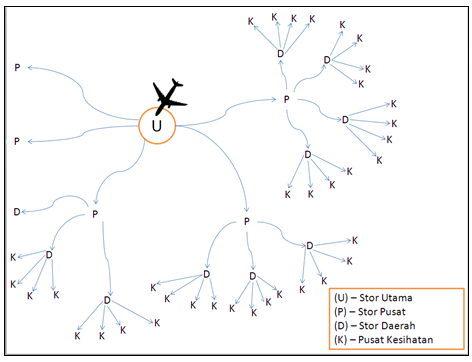 Image 1: Distribution Chain for Vaccines into Malaysia
Image 1: Distribution Chain for Vaccines into Malaysia
Vaccines imported from overseas usually reached through Kuala Lumpur International Airport Cargo and transfer straight to Central Store. From the Central Store the vaccines will be distributed to the Regional Store followed by the District Store and finally reaches the Health Centres. Dedicated transports like refrigerated trucks are used during the distribution of the vaccines between the centres and stores. Distributing parties hold the responsibilities to ensure best delivery route is planned out before hand before the actual delivery. This series of cold chain handling need to be strictly controlled to ensure that the vaccine is still in acceptable condition before it can be administered to the patient.
How did they do it?
Only dedicated trained personnel are assigned to handle cold chain goods. Given the storage temperature of most vaccines have to be within a narrow range of 2-8 degree Celsius, those personnel are specifically trained to understand the product physical properties, packing requirements, handling procedures and transportation of the vaccines ensuring every step are carried out appropriately. The handling of vaccines starts from the manufacturing stage of the vaccines till the final administration of the vaccine on to the patients, the temperature of the storage and transportation have to be monitored throughout on a continuous daily basis using temperature loggers. The following images are examples of temperature monitoring control/device used:
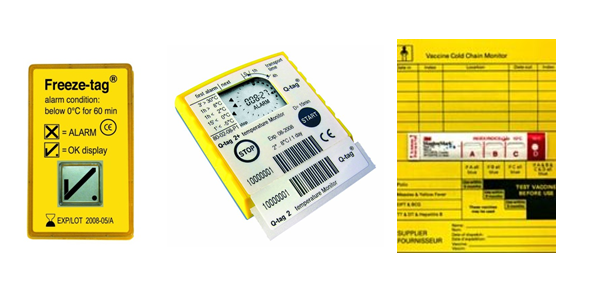 Image 2: Examples of Temperature Monitoring Device (Freeze-tag, Q-Tag2+, CCM Card)
Image 2: Examples of Temperature Monitoring Device (Freeze-tag, Q-Tag2+, CCM Card)
Image Source: http://www.berlinger.ch/
Vaccines in Malaysia are regulated by the National Pharmaceutical Control Bureau (NPCB) and the storage and transportation of the vaccines are subject to the requirements stated in the Guidelines on Good Distribution Practice (GDP), Malaysia. On top of that, vaccines importations to Malaysia have to undergo the Vaccine Lot Release which is fully enforced on 1st February 2015 (www.bpfk.gov.my).
What can you do?
Vaccines are medicines which can be life-saving or even prevent contagious infectious diseases. Its function and uses are approved by the Ministry of Health, Malaysia ensuring the safety, efficacy and quality of the medicine is maintained at all time. Therefore, before the administration of vaccine, you can always perform the followings:
- Check the correct storage condition of the vaccine;
- Ensure the expiry date of the vaccine labels is still within the expiry date
If you find any discomfort or side effects or worsening of the condition after an administration of vaccine, do report it immediately to your nearest health care centres for further assistance.
References:
- Cold Chain. ISML.org Primary Care Support. Immunisation.
- Malaysian Good Distribution Practice (GDP) Guidelines; 2nd Edition; 2013.
- WHO. Annex 9 Model guidance for the storage and transport of time- and temperature–sensitive pharmaceutical products. WHO Technical Report Series, No.961, 2011.
- Childhood Immunisation. Clinical Practice Guidelines. MOH/P/PAK/81.04 (GU).
- WHO: Aide-memoire for prevention of freeze damage to vaccines. WHO/IVB/07.09.
- WHO: Safe vaccine handling, cold chain and Immunizations. WHO/EPI/LHIS/98.02.
- Berlinger Products and Technology. http://www.berlinger.ch
- Vaccine Lot Release in Malaysia. www.bpfk.gov.my
- National Pharmaceutical Control Bureau Portal. www.bpfk.gov.my
| Last Reviewed | : | 13 November 2015 |
| Writer/Translator | : | Cheok Xin Yin |
| Accreditor | : | Ahmad Syamsury bin Sulaiman |