What is the Medicine Advertisements Board (MAB)?
Have you seen a Kementerian Kesihatan Lembaga Iklan Ubat (KKLIU) approval number which has a 4-digit numbers followed by the year it was approved as follows “KKLIU 0123/2015”? KKLIU numbers will be displayed in the advertisement of medical products or healthcare facilities and services published either in print or electronic media. By the way, do you know who is the authority responsible for approving this KKLIU?
Example of an approved advertisement:
Members of the Medicine Advertisements Board
The authority responsible for approving medical products advertising as well as the advertising of healthcare facilities and services is the Medicine Advertisements Board (MAB). The Board was established under the provisions of the Medicine Advertisements Board Regulations 1976. Establishment and composition of the MAB members are as below:
- The Director-General of Health, Ministry of Health Malaysia who shall be an ex-officio member
- The Director of Pharmaceutical Services, Ministry of Health Malaysia who shall be an ex-officio member
- The Director of Medical Services, Ministry of Health Malaysia who shall be an ex-officio member
- The Secretary, Malaysian Medical Association who shall be an ex-officio member
- The Secretary, Association of Private Hospitals of Malaysia who shall be an ex-officio member
- The Secretary, Federation of Private Medical Practitioners Association who shall be an ex-officio member
- One physician in the public service to be appointed by the Minister
- One pharmacologist to be appointed by the Minister
- One pharmacist in the public service to be appointed by the Minister
- One officer from the Ministry of Information nominated by the Minister of Information to be appointed by the Minister
Roles and Responsibilities of the Medicine Advertisements Board
The Board was established in order to discuss the approval for application pertaining to medical products or healthcare facilities and services advertisement received and at its discretion, the Board may:
- Approve or reject the advertisement application for MAB’s approval
- Review and revise the MAB policies and advertising guidelines when necessary
- Cancel or withdrawal of approved advertisements
Client’s Charter
Each application for MAB’s approval submitted by advertisers will be reviewed and evaluated by the Secretariat of MAB. The issuance of approval by the MAB is within 5 working days upon receiving complete application and provided that the advertisement format complies with the MAB guidelines and policies or within 5 working days after the MAB meeting. The application form for MAB’s approval as well as the MAB advertising guidelines and policies are all available on the Pharmaceutical Services Division website: www.pharmacy.gov.my
What If The Applicant Is Not Satisfied With the MAB’s Decision?
Pursuant to Section 6 of the Medicine Advertisements Board Regulations 1976: Any person aggrieved by any decision of the Board may appeal to the Minister of Health whose decision shall be final. All the appeal notice must be made within 14 days from the date of the decision by the MAB. A 30-day period is given for submission of additional data or information or any document for the purposes of the appeal. An appeal will not be entertained if all the required information is not submitted within the time frame stipulated.
Statistic
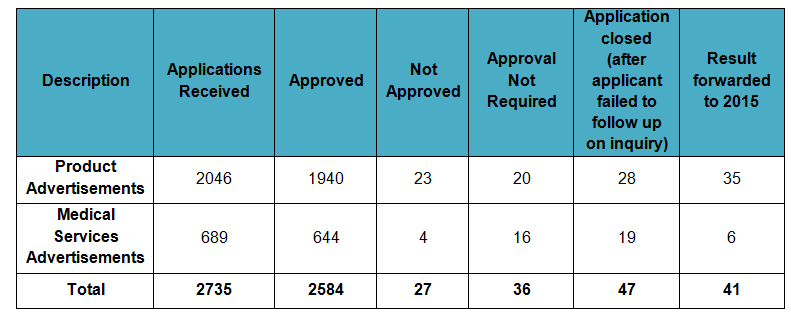
Statistics of advertisement applications for 2014 are as shown in Table A. Advertisement approvals for year 2012-2014 are shown in Table B.
TABLE A: Advertisement Applications for 2014
TABLE B: Advertisement Approvals for Years 2012 – 2014
|
Description/Year
|
2012
|
2013
|
2014
|
|---|---|---|---|
| Total number of applications | 2133 | 2562 | 2735 |
| Total number of applications processed within client charter period | 2078 | 2317 | 2594 |
| Total number of applications processed outside of client charter period | 55 | 245 | 141 |
| Percentage of applications approvals within client charter period (Target > 90%) | 97.4% | 90.4% | 94.8% |
References
- Website www.pharmacy.gov.my
- Medicine Advertisements Board Regulations 1976
| Last Reviewed | : | 05 June 2015 |
| Writer/Translator | : | Wong Hui Shean |
| Accreditor | : | Umikalsom bt. Ibrahim |