PET-CT (Positron Emission Tomography-Computed Tomography) is a modern nuclear imaging technique that is useful to investigate functional imaging related to the cancer disease. The thyroid, bones, heart, liver and many other organs can be easily imaged, and disorders in their function revealed by using Positron Emission Tomography (PET) radiopharmaceutical. The unique character of PET radionuclide, its produce Positrons (?+) with different energy up to 3.5 MeV. PET radionuclide is unstable elements and after losing all of its kinetic energy, it interacts with an electron and annihilated process will occur. Both the positron and electron exist inside patients body are converted to gamma energy during annihilation and simultaneously two 511 KeV photons are emitted at opposite direction to each other. The PETcamera is based on the coincidence detection of the two aforementioned photons. Coincidence detection is a powerful method enhancing sensitivity and dynamic-imaging capabilities of PET. PET-CTcamera systems contain a ring of detectors that encircles the patient body. The annihilation gamma ray were collected over many angles around the body axis of the patient is used to reconstruct the image of the activity distribution in slice or tomographic form.Basically, there are three major disciplines that have to interact and collaborate closely to for application of PET in a clinical setting such as medical physics, radiopharmaceutical sciences and clinical imaging field.Each discipline covering its specific field in depth to enable the successful of PET-CT imaging. Hence, understanding of radiopharmaceutical issues is very important to understand nuclear medicine imaging on a molecular level.
In general the development of the PET radiopharmaceutical has to be based on the following considerations:
- Availability and the suitability of the radionuclide to the human (the cyclotron facility to produce PET radionuclide);
- Physical characteristics of the radionuclide (prefer short half live due to radio toxicities issues)
- Radiochemicallabeling issues (low residual chemical compound and radiochemical purity);
- Radiopharmaceutical kinetic in human (radio toxicities issues)
Currently there are several PET radioisotopes that are suitable to be used as radiopharmaceutical as shown in Table 1. Principally, a PET radiopharmaceutical involving of two components such as a molecular structure such as vector, vehicle and ligand and second components is a source of PET radionuclide. Some cases the connection of these two components, generally called linker may be necessary. The vehicle defines the biological characteristics and is responsible for chemical and biochemical interactions within the living organism as shown in Figure 1.
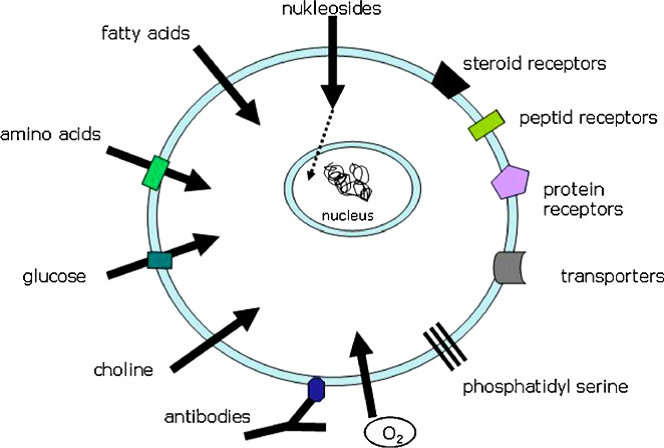
Image source: www.sciencedirect.com
Figure 1: Main targets for radiopharmaceuticals on the cell surface (Source:Basics and principles of radiopharmaceuticals for PET/CT)
|
PET radioisotope
|
Max ? Energy (MeV)*
|
Produce by | Half Life |
Usage
|
|---|---|---|---|---|
| 18F | 0.633 (96.7%) | Cyclotron | 110 min | Abnormal glucose uptake in oncology |
| 11C | 0.980 (99.8%) | Cyclotron | 20.4 min | Prostate cancer |
| 13N | 0.492 (99.8%) | Cyclotron | 9.97 min | Cardiology |
| 15O | 0.735 (99.9%) | Cyclotron | 122 sec | Brain blood flow volume |
| 124I | 1.532 (11%) 2.135 (12%) |
Cyclotron | 4.2 days | Thyroid uptake |
| 86Y | 1.253 (12.4%) | Cyclotron | 14.7 days | Bone pain palliation agent |
| 68Ga | 1.899 (88%) | Generator | 1.13 hour | Neuroendocrine |
| 82Rb | 3.5(95.5 %) | Generator | 1.237 min | Cardiology |
Table 1: Common radionuclide in PET imaging.
PET radiopharmaceutical properties can be easily substituted directly into biomolecules without changing the properties of the molecule. Currently, more than 95 % of all PET imaging studies were performed by utilize an analog of glucose, 18F-2-fluoro-2-deoxy-d-glucose (F18-FDG). The use of F18- FDG to image glucose metabolic rate takes advantage of the observation, that malignant cells have higher rates of aerobic glycolysis than other normal tissues. F18-FDG radiopharmaceutical also has been confirmed as the new standard for staging patients with Hodgkin lymphoma (HL) as well as for assessment of treatment response and should be replaced contrast-enhanced CT for most patients.F18-FDG also useful for studying myocardial viability, due to the greater utilization of glucose by the proliferating cells. A number of other F-18 labelled radiopharmaceuticals are being developed and a few of them are still under research as shown in Table 2. Since its development for more than 40 years ago, 18F-FDG had start the influence on research in the PET imaging. F18-FDG is now the standard radiotracer used for PET neuroimaging and cancer patient management due to its property. In addition to infection imaging, Ga68 is finding use in cancer imaging when labelled with peptides. The ultra short-lived Rb82 (a half-life of 75 seconds), available from a Strontium-82 – Rb82 generator, and useful for PET imaging of blood flow to myocardium, has high potential in managing heart patients. There is a series of PET tracers that found their way into clinical routine so far.
|
F18 tracer
|
Specific remarks
|
|---|---|
| F18-FDOPA | Useful diagnosis of Parkinson’s disease. Now, extended into neuroendocrine tumor imaging. |
| F18-FET | Able distinguish between inflammation and residual tumor tissue. A good alternative to replace authentic C11 and longer half-life. |
| F18-FMISO | Gold standard amongst tracers for hypoxia imaging. |
| F18-FLT | Gold standard for uptake reflects thymidine kinase activity. |
| F18-Choline | Longer-lived alternatives for authentic C11-Choline and C11-Acetate |
| F18-FAZA | Chemical derivative of FMISO bearing aarabinoses-backbone. So far, only used in clinical trials. |
| F18-EF-5 | So far, only used in clinical trials. |
| F18-FES | Used for the diagnosis of estrogen receptor positive tumors (e.g. breast). |
Table 2: Common F18 labelled with pharmaceutical agent
The radionuclide with its specific physical properties is the working basis for signal detection for localization of the target tumor or specific organ. On the other hand, understanding the (bio-) chemical properties of the molecular vehicle such as binding characteristics, metabolism, and elimination rate is essential for molecular modelling and dosimetry. From a clinical point of view, basic understanding of radiochemical preparation such as formation of potential by-products, interference of labelled and unlabeledradiopharmaceuticals to the target site and specific radioactivity is also necessary for diagnosis using PET-CTcamera.Currently, there are four PET radiopharmaceuticals officially recognized by FDA: sodium fluoride (F18-Na) for bone imaging, rubidium chloride (82Rb-Cl) for assessment of regional myocardial perfusion in the diagnosis and localization of myocardial infarction, F18-FDG for identifying the regions of abnormal glucose metabolism and primary and metastatic malignant diseases and ammonia (13N-H3) for assessment of myocardial blood flow. PET imaging requires expensive equipment’s including a cyclotron for PET radionuclide production, automated chemistry devices for PET labelling procedure, purification instrumentation, and PET-CT camera. Though there are applications of PET imaging in cardiology and neurology, it seems that its greatest health application and better benefit is in cancer diagnosis.
PET radiopharmaceutical are a very special category of pharmaceutical drugs in most of the country in the world. This has consequences both in preparation and regulatory treatment due to radionuclide properties. Delivery of PET radiopharmaceutical more than four hours is a big issues due to short-lived radionuclides and not recommended for very long duration of transportations. Following the radiolabelling procedure, the possible changes in the in vivo behavior of PET tracers have to be taken into account. Overall, PET radiopharmaceutical are the heart of molecular imaging, pumping life into PET-CT imaging and the future of PET radiopharmaceutical is open for further explorations.
References
Wadsak W, Mitterhauser M, Wadsak W, et al. Basics and principles of radiopharmaceuticals for PET/CT. Eur J Radiol. 2010;73(3):461-469.
| Last Reviewed | : | 31 October 2017 |
| Writer | : | Mohd Aminuddin bin Said |
| Accreditor | : | Mohd Hizwan bin Mohd Yahya |







